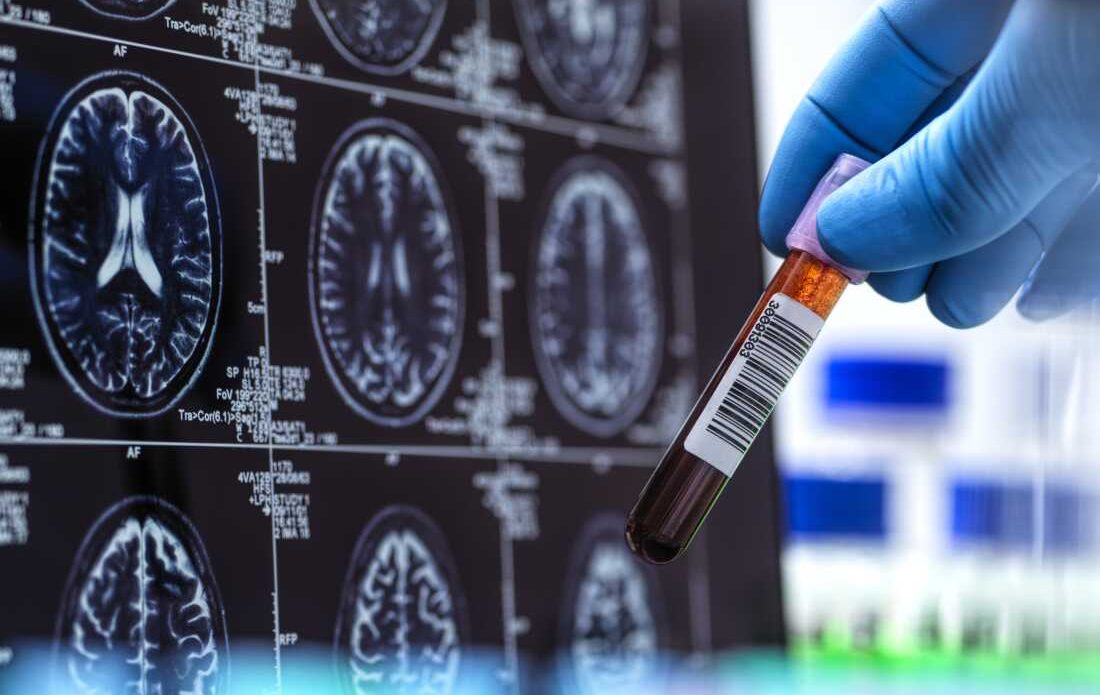
In a groundbreaking advancement, U.S. health authorities have approved the first-ever blood test designed to help diagnose Alzheimer’s disease. The test, greenlit in mid-May, could allow patients to begin treatment earlier—potentially slowing the progression of this debilitating neurodegenerative disorder.
Developed by Fujirebio Diagnostics, the test works by measuring the levels of two specific proteins found in the blood. These protein levels are associated with the presence of beta-amyloid plaques in the brain, a hallmark of Alzheimer’s disease. Previously, such biomarkers could only be detected through expensive brain scans or invasive spinal fluid analysis.
“This is a significant development,” said Dr. Marty Makary of the U.S. Food and Drug Administration (FDA). “Alzheimer’s affects more people than breast and prostate cancer combined. Currently, about 10% of individuals aged 65 and older live with the disease, and that number is projected to double by 2050.”
Currently, two FDA-approved drugs—lecanemab and donanemab—target these amyloid plaques and have shown modest success in slowing cognitive decline. While they do not cure the disease, supporters, including many neurologists, believe the drugs can extend patients’ independence by several months, particularly when administered early.
Clinical trials of the new blood test revealed results closely aligned with more traditional methods like positron emission tomography (PET) scans and cerebrospinal fluid analysis. The test is now approved for clinical use in patients showing signs of cognitive decline, though doctors are advised to consider the results in conjunction with other clinical information.
Dr. Michelle Tarver of the FDA’s Centre for Devices and Radiological Health called the approval “an important milestone in Alzheimer’s diagnostics.” She added that the new tool will make early detection more accessible to patients across the United States.
Alzheimer’s disease is the most common form of dementia. It progresses gradually, leading to memory loss, reduced cognitive function, and eventually, loss of independence. With early diagnosis now more feasible, patients and their families may have a better chance to plan for and manage the challenges of the disease.